Oil spill cleanup experiment
Very first, some background info on oil and liquid...
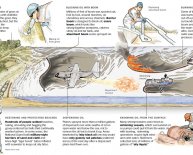
There were numerous oil spills over time, like the Gulf oil spill within the U.S. that occurred in April 2010, when there clearly was any sort of accident at a drilling rig when you look at the Gulf of Mexico. A spill similar to this which close to land triggers many dilemmas when it comes to environment and makes it difficult to cleanup. As low as three gallons of oil can distribute to create a slick mess covering one acre of ocean's surface. Using the Gulf oil spill, it is projected that 200, 000 gallons everyday have spilled to the sea as researchers work to stop the leak and cleanup.
Oil spills similar to this are extremely harmful, however they aren't the only real source of oil which polluting our seas. Rain washes particles from air pollution into the sea. Normal seeps from the sea base additionally occur on a yearly basis. And something of the biggest types of oil polluting the sea is thought becoming from the oil folks put down their particular empties every day, as well as the runoff from parking lots. So, cleaning the sea is a vital concern, not merely as a result of recent oil spills.
Oil and liquid never combine — while you might already know just. You understand that oil is sticky and oily. This will make it challenging clean up. Why don't we take a look at the substance properties of oil and water to see why.
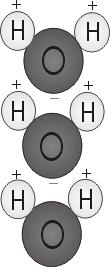
Each liquid molecule consists of two hydrogen atoms plus one air atom — H2O. If the two hydrogen atoms bond using the air, they attach to the top of the molecule instead like Mickey Mouse ears. This molecular framework gives the liquid molecule polarity, or a lopsided electric cost that attracts various other atoms. The termination of the molecule using two hydrogen atoms is favorably recharged. The other end, with all the oxygen, is negatively charged. Like in a magnet, in which north poles tend to be drawn to south poles ("opposites attract"), the good end of the liquid molecule will relate genuinely to the negative end of other molecules. Because of their polarity, water molecules are strongly attracted to one another.
What happens when oil and liquid are put collectively? Both liquids will not combine. Oil is non-polar, meaning its particles are balanced, with equal sharing of electric particles, rather than one negative plus one good end. Regardless of how much you stir it, a variety of oil and water don't bond, due to the fact molecules can not link.
Oil is constructed of more complicated particles, containing carbon and hydrogen. Oil particles tend to be non-polar, indicating they do not stick collectively like water molecules do. Oil is dense and hefty, yet its particles tend to be spread farther apart, lowering the density. Since it has a diminished density, oil floats on water's surface. Oil can be slippery — it doesn't stick together just like the polar molecules of liquid.
Experiment 1 – what the results are when oil is added to liquid?
You understand that water and oil do not blend. Take a look at just how they react to both and discover what the results are to generate an oil slick.
- Fill an obvious synthetic container just a little over halfway up with liquid.
- Pour some falls of preparing oil to the water, watching what happens when you begin pouring very gradually, then afin de quickly.
The oil always floats into the top of the liquid in the container. Even if you had been to release it in the bottom, it can float up. If you afin de slowly, the oil will stick collectively, making a large puddle. In the event that you pour faster, the oil pauses into droplets, or smaller puddles. In the event that you look closely, you can observe tiny drops or particles of oil that are drifting at first glance. A few of these traces of oil — huge and small — make an oil slick. Sometimes the oil is spread acutely slim, and in various other spots discover a messy oil puddle. An oil slick is done whenever plenty of oil is circulated, so it floats to the surface and spreads over a large location. Inside ocean, waves in addition to wind distribute the oil around much more.
Test 2 – Oil cleaning making use of absorbents
Reuse the container of liquid and oil through the final research. Get a hold of some materials around your house or school having absorbent properties and could help absorb the oil to wash within the oil slick. Some situations: cotton fiber balls, washing lint, a paper towel collapsed into strips, and makeup products remover pads or sponges. Ensure you choose something small and light.
- Place one of the absorbent items in to the container of water, moving it around to soak up the spill.
- Try containing the oil by blocking off a small location with a few bits of the absorbent product. Can you make a square across the spill, without any oil escaping?
- Try out this project once again using among the various other absorbents. (you have made have to add more oil into the liquid to generate more slicks.)
- Once the greater part of oil is absorbed, dispose of the absorbent pads and just about every other products you tried after which afin de water along the sink.
In actual life, drifting objects, sort of like a big sock, are carried to the ocean then forced from the part of a vessel to simply help take in oil spills. These socks (called booms) tend to be filled with absorbent material that will absorb plenty, yet are also lightweight. You created a kind of mini increase inside research. It works to wash up liquid by soaking-up the messy spill once it gets close to a drop of oil. These booms are very effective, but the difficulty is in controlling them so that they go where the oil is. Was it tough to support the oil to a little location inside experiment? Many little falls escaped, didn't they? Imagine how tough blocking off a location with booms would-be for a real oil spill into the sea which has waves and wind. You'll increase this science project further by seeing just how much oil each absorbent material can contain, or the length of time they can sit in a container of water before soaking it and having wet throughout. For greater outcomes, be in line with the size of pieces you utilize, and time your experiments utilizing a stopwatch. Record the outcome of your experimenting by keeping a journal on lined report or a notebook. You may also draw maps or graphs for your laboratory log.
Oil Spill Cleanup Experiment System
Might like to do more detailed science projects? This new kit was created to instruct students exactly about oil spills. It offers all products you need (except for water) plus full guidelines for doing six different experiments. Learn about the polarity of liquid, use feathers and sponges to study the consequences of crude oil on pet life additionally the environment, and try out genuine techniques that are used to clean up an oil spill (such as absorption and dispersion). Includes tips for research reasonable tasks. Ages 10 & up.